Page 223 - 《精细化工》2023年第4期
P. 223
2+
す 4 ⍖ 㤺喑ぶ: Ά䚥䨹ᩦᕔ⽨ภ⩌➖◚⩢ღࣷ⩢॥䭱 Cu ᕔ㘪 g909g
➖◚ᱽ᫆ȡ䕇䓴 SEM जВⰸݝ喑ᩦᕔ⩌➖◚㶕䲏㇄
㈆Ƞႁ䯆ࣾ䓫́䭱Ɑ⮪㞟䷄ㆿȡXRD 䅞जⰸܧ喑㶕
䲏䉌䒪⮱⮪㞟䷄ㆿ᭜क़䨹⮱⅔ࡃ➖ȡ
喍2喎ᩦᕔ⽨ภ⩌➖◚जᒏᰡ็ႁ䯆㐀Ჱ喑℁
㶕䲏⼜๔喑ϸႁߍȡ⩞λڤᰶ๔⮱℁㶕䲏⼜হ
ᰡ็⮱ϸႁ喑䔆जВҬ MRHC ⩢Ხڤᰶ℁ RHC ⩢
Ხᰡ倅⮱℁⩢ღহᰡхᐯ⮱䕌⢴ᕔ㘪ȡ
喍3喎̻᱗ᩦᕔ⽨ภ⩌➖◚Ⱕ℁喑ᩦᕔऻ⩌➖◚
℁⩢ღᬻ᭫倅喑⩢䭨᭫㦄ۼᄼ喑ᓗ⣜ᕔ㘪হԺ⢴
2+
ఫ 10 Cu ⊀Ꮣᄦ⩢॥䭱᩵⮱ᒞ৺ ᕔ㘪ᰶࡴ喑́ᒀΆ䚥䨹⊀Ꮣͧ 0.3 mol/L ᬣ喑
2+
Fig. 10 Effect of Cu concentration on electrosorption effect MRHC-0.3 ⩢Ხౕ 0.5 A/g ᬣ喑℁⩢ღ䓫 191.94 F/g喑
ౕ2 A/g̸ٲᩫ⩢2000⁎ऻ喑ڣ℁⩢ღԊᠮ⢴ͧ92.16%喑
3.4 ⩢Ხ⮱ں⩌ᕔ㘪
倅 λ᱗ᩦᕔ ⽨ภ⩌➖ ◚⩢Ხ℁ ⩢ღԊᠮ ⢴
⩢॥䭱ឭᱜᰭ๔х߬᭜⩢Ხ䓫ݝ॥䭱亞হऻज
喍75.81%喎喑ᓗ⣜⽠Ⴧᕔ㘪㞜ສȡ
䕇䓴㙞䭱⣝⩢Ხ⮱ᓗ⣜ں⩌ȡ䞡λⴚᣒ㙞䭱᧺҉
喍4喎ᩦᕔ⽨ภ⩌➖◚⩢Ხ MRHC-0.3 ⩢॥䭱
ᰡӬ喑䓽㵹᱙䒰ѻ喑᱙侹䔶⩕ⴚᣒ㙞䭱䔈㵹
2+
Cu ⮱ᰭҠ⩢ࢸͧ 0.9 VȠᰭҠ pH ͧ 5ȡౕᰭҠ
⩢Ხ⮱ᓗ⣜ں⩌喑㐀㻮ఫ 11ȡຯఫ 11 喑⩢Ხ
2+
2+
У̸⩢॥䭱 Cu 喑Cu ݊䉕䛼⊀Ꮣͧ 50 mg/L
ౕす̭⁎ᓗ⣜㙞䭱ऻ⏣⋟⩢ᄩ⢴⇎ᰶఋݝ݊թ喑䔆 2+
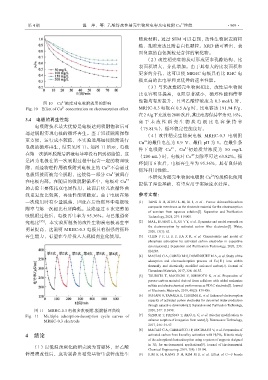
喍200 mL喎ᬣ喑⩢Ხᄦ Cu ࣨ䮑⢴ज䓫 63.82%ȡᓗ
᭜ͧ⩢Ხౕす̭⁎॥䭱䓴⼸͚࠲क़̭Ⴧ⮱➖⤳॥ ⣜Ҭ⩕ 6 ⁎ऻ喑⩢Ხں⩌⢴ͧ 95.36%喑ڤᰶ㞜ສ⮱
2+
䭱喑㔹㏼➖⤳҉⩕㷘॥䭱ݝ⩢Ხ̷⮱ Cu ̺ч䕇䓴 ᓗ⣜ݖ⩕ᕔ㘪ȡ
2+
⩢Ხⴚᣒ㔹㷘Ⴙڕ㙞䭱喑䔆Ҭᓄ̭䘕ܳ Cu 㷘⪆ႅ ᱙ⵁ⾣ͧ⽨ภ⩌➖◚⩢॥䭱 Cu ⮱㻱ࡃҬ⩕
2+
ౕ⩢Ხڲ䘕ȡౕ䮼ऻ⮱॥㙞䭱ᓗ⣜͚喑⩢Ხᄦ Cu 2+
ӈγ⤳䃧ധ喑ᰶ᱈Ꮑ⩕λ䭲ᏌⅡั⤳ȡ
⮱ࣨ䮑ͨ㺮ӊចࣹ⩢ᅯ҉⩕喑ᩲ㔹ऻ㐚܍⁎ᓗ⣜ᰟ
㏬䛺ฺᏓ℁䒰倅喑ں⩌ᕔ㘪ᰡ⽠Ⴧȡ⩞λ⩢Ხౕす ࣯㔰᪴⡛喟
̭⁎Ҭ⩕ᬣᰶᄾ䛼㙞㥪喑ₑౕऻ㐚ᓗ⣜͚⩢Ხ॥ [1] TANG X H, ZHOU L M, XI J, et al . Porous chitosan/biocarbon
䭱⢴̻す̭⁎Ⱕ℁ᰶ䭺ѻȡᰭ㏵㏼䓴 6 ⁎Ⴙ᪡⮱ composite membrane as the electrode material for the electrosorption
of uranium from aqueous solution[J]. Separation and Purification
॥㙞䭱䓴⼸ऻ喑⩢Ხں⩌⢴ͧ 95.36%ȡ̻ጟ្䖀ⵁ Technology, 2021, 274: 119005.
⾣Ⱕ℁ [35] 喑᱙侹ݣิ⮱ᩦᕔ⩌➖◚⩢Ხں⩌⢴ [2] MA L, HUANG L Y, XU Y Y, et al. Dynamics and model research on
the electrosorption by activated carbon fiber electrodes[J]. Water,
ᬻ᭫倅喑䔆䄡ᬻ MRHC-0.3 ⩢Ხڤᰶᒵᑧ⮱ᓗ⣜ 2020, 13(1): 62.
ں⩌㘪߈喑ᰶ᱈ౕϷऻែڒ๔㻱ੳ͇ࡃҬ⩕ȡ [3] CHEN F F, LI H F, JIA X R, et al. Characteristic and model of
phosphate adsorption by activated carbon electrodes in capacitive
deionization[J]. Separation and Purification Technology, 2020, 236:
116285.
[4] MACIAS G A, CORZO M G, DOMINGUEZ M A, et al. Study of the
adsorption and electroadsorption process of Cu(Ĕ) ions within
thermally and chemically modified activated carbon[J]. Journal of
Hazardous Materials, 2017, 328: 46-55.
[5] TSUBOTA T, MAGUCHI Y, ISHIMOTO K, et al. Preparation of
porous carbon material derived from cellulose with added melamine
sulfate and electrochemical performance as EDLC electrode[J]. Journal
of Electronic Materials, 2019, 48(2): 879-886.
[6] SUFIANI O, TANAKA H, TESHIMA K, et al. Enhanced electrosorption
capacity of activated carbon electrodes for deionized water production
through capacitive deionization[J]. Separation and Purification Technology,
ఫ 11 MRHC-0.3 ⩢Ხ็⁎॥䭱-㙞䭱ᓗ⣜ᰟ㏬ 2020, 247: 116998.
Fig. 11 Multiple adsorption-desorption cycle curves of [7] SIZMUR T, FRESNO T, AKGUL G, et al. Biochar modification to
MRHC-0.3 electrode enhance sorption of inorganics from water[J]. Bioresource Technology,
2017, 246: 34-47.
[8] MACIAS G A, CARRASCO J P, ENCINAS S V, et al. Preparation of
4 㐀䃧 activated carbon from kenaf by activation with H 3PO 4. Kinetic study
of the adsorption/electroadsorption using a system of supports designed
in 3D, for environmental applications[J]. Journal of Environmental
喍1喎Вѻ⍖䶱◚ࡃ⮱⽨ภ◚ͧݺ侞Ҁ喑㏼Ά䚥
Chemical Engineering, 2019, 7(4): 103196.
䨹⊥⌺ᩦᕔऻ喑ߌݣิܧ⽨ภധ㵺⩌䒪䨹ᩦᕔ⩌ [9] KIM K H, KANG D H, KIM M J, et al. Effect of CÿF bonds