Page 158 - 《精细化工》2023年第4期
P. 158
g844g ㏳ࡃጒ FINE CHEMICALS す 40 ࢤ
DPPH-A-PPEK1 ⮱㉜⼜䉕䛼ᢌ⢴ᰭᄼ喑ͧ 3.1%ȡ ᐯ⮱☚⽠Ⴧᕔ喑ज䖬ٺᰶᱧᄼܳၽధࡃݯౕ PN
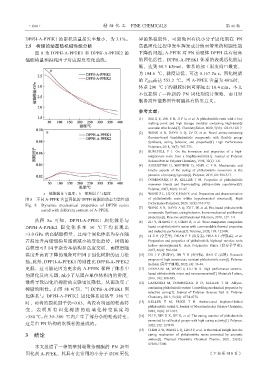
2.5 ᴾ㘯⮱ߕᔮ☚ᱧᷝᕔ㘪ܳᲽ 倅⍖ధࡃ䓴⼸͚ࣾ⩌ᡒࣾᝃܳ㼐㔹ፓᲒ⮱ᴾ㘯ᕔ㘪
ఫ 8 ͧ DPPH-A-PPEK1 হ DPPH-A-PPEK2 ⮱ ̸䭺⮱䬛䷅ȡA-PPEK ᄦ PN ݺ侞Ҁ DPPH ڤᰶ䒰倅
ו㘪䛼হᢌ㕄ၽᄦᏁ⍖Ꮣअࡃᰟ㏬ȡ ⮱ధࡃ≨ᕔ喑DPPH-A-PPEK1 Ҁ㈨⮱㶕㻯≨ࡃ㘪ᰭ
ѻ喑䓫ݝ 68.3 kJ/mol喑Ҁ㈨⮱ߍጒ⍖Ꮣ⿄ऐᰭც喑
ͧ 194.6 Ď喑叼Ꮣᰭѻ喑ज䓫 0.167 Pa·s喑ధࡃᴾ㘯
⮱ T d5% 倅䓫 553.2 Ďȡᒀ A-PPEK क़䛼ͧ 40%ᬣ喑
Ҁ㈨ 200 Ď̸⮱܊㘣ᬣ䬡ज㑖ⴚ㜠 10.4 minȡ᱙᪴
̺ϲӈγ̭⮱ PN ధࡃݯ䃫䃎ゃ⪒喑㔹́ᄦ
ݣิ倅ᕔ㘪☚ధᕔᴾ㘯ڤᰶՌ䞡ᘼͶȡ
࣯㔰᪴⡛喟
[1] SHI X Y, BAI S N, JI P G, et al. A phthalonitrile resin with a low
melting point and high storage modulus containing high-density
aromatic ether bonds[J]. ChemistrySelect, 2020, 5(39): 12213-12217.
[2] WANG A R, DAYO A Q, LV D, et al. Novel amino-containing
fluorene-based bisphthalonitrile compounds with flexible group:
Synthesis, curing behavior, and properties[J]. High Performance
Polymers, 2018, 30(7): 767-775.
[3] BURCHILL P J. On the formation and properties of a high-
temperature resin from a bisphthalonitrile[J]. Journal of Polymer
Science Part A: Polymer Chemistry, 1994, 32(1): 1-8.
[4] AUGUSTINE D, MATHEW D, NAIR C P R. Mechanistic and
kinetic aspects of the curing of phthalonitrile monomers in the
presence of propargyl groups[J]. Polymer, 2015, 60: 308-317.
[5] DOMINGUEZ D D, KELLER T M. Properties of phthalonitrile
monomer blends and thermosetting phthalonitrile copolymers[J].
Polymer, 2007, 48(1): 91-97.
aÿו㘪䛼̻⍖Ꮣ喠bÿᢌ㕄ၽ̻⍖Ꮣ [6] WANG Z L, LIU X Y, HAN Y, et al. Preparation and characterization
ఫ 8 ̺ह A-PPEK क़䛼ధࡃ⮱ DPPH ᴾ㘯⮱ߕᔮ߈႓ᕔ㘪 of phthalonitrile resin within hyperbranched structure[J]. High
Fig. 8 Dynamic mechanical properties of DPPH resins Performance Polymers, 2020, 32(8): 963-972.
cured with different content of A-PPEK [7] WANG A R, DAYO A Q, ZU L W, et al. Bio-based phthalonitrile
compounds: Synthesis, curing behavior, thermomechanical and thermal
properties[J]. Reactive and Functional Polymers, 2018, 127: 1-9.
ϻఫ 8a जⴒ喑DPPH-A-PPEK1 ధࡃҀ㈨̻ [8] ZU Y, ZHANG F F, CHEN D, et al. Wave-transparent composites
DPPH-A-PPEK2 ధࡃҀ ㈨ౕ 50 Ď ̸ 㶕⣝ܧ based on phthalonitrile resins with commendable thermal properties
and dielectric performance[J]. Polymer, 2020, 198: 122490.
>3.0 GPa ⮱倅ו㘪䛼喑́͑͗ధࡃҀ㈨ႅౕ䮼 [9] LI Z H (ᱻ㟊ࡻ), DUAN F F (⃢㟠㟠), HUA S J (ࡻଶ), et al.
Ɑ⍖Ꮣࡴ倅ו㘪䛼䔽⌽ۼᄼ⮱अࡃ䊸߬喑ᴾ㘯⮱ Preparation and properties of phthalonitrile biphenyl novolac resin
hollow microspheres[J]. Acta Polymerica Sinica (倅ܳၽ႓្),
倅䛼जᑿλᱯ㟠ധ㐀Ჱহ倅Ꮣϑ㖁喑㔹䛼䮼 2017, 48(4): 596-604.
⍖Ꮣࡴ倅㔹̸䭺⮱⣝䆎जᑿλధࡃᴾ㘯⮱Ꮑ߈Ძ [10] DU J Y (⦫Ⴔ), JIN Y H (䛾ႴᲚ), GAO C (倅ྡྷ). Research
progress of high temperature resistant phthalonitrile resin[J]. Polymer
ᑈȡₑใ喑DPPH-A-PPEK1 ⮱݇ᕔ℁ DPPH-A-PPEK2
Bulletin (倅ܳၽ䕇្), 2022, (4): 38-44.
ᰡѻ喑䔆ज㘪᭜ͧᰡ็⮱ A-PPEK ⼭䛷γҀ㈨͚ [11] DERRADJI M, WANG J, LIU W B. High performance ceramic-
⮱ధࡃࣺᏁധఏ喑ۼᄼγധఏౕࢂѺҀ⼜ڲ⮱ჳᏓ喑 based phthalonitrile micro and nanocomposites[J]. Materials Letters,
2016, 182: 380-385.
ᰭ㏵ᄩ㜡ధࡃऻᴾ㘯⮱ϑ㖁ჳᏓ䭺ѻ喑ϻ㔹ᩦअγ [12] LASKOSKI M, DOMINGUEZ D D, KELLER T M. Alkyne-
ᴾ㘯⮱݇ᕔȡ⩞ఫ 8b जⴒ喑ᒀ DPPH-A-PPEK1 ధ containing phthalonitrile resins: Controlling mechanical properties by
selective curing[J]. Journal of Polymer Science Part A: Polymer
ࡃҀ㈨̻ DPPH-A-PPEK2 ధࡃҀ㈨ߍ☚㜠 380 Ď Chemistry, 2013, 51(22): 4774-4778.
ᬣ喑͑㔲⮱ᢌ㕄ၽϺ<0.03喑⇎ᰶᬻ᭫⮱Ძᑈ䒙 [13] KELLER T M, PRICE T R. Amine-cured bisphenol-linked
phthalonitrile resins[J]. Journal of Macromolecular Science-Chemistry,
अ 喑㶕ᬻ ᰶ ధࡃᴾ 㘯⮱ ⣨⦰ࡃ 䒙अ ⍖Ꮣ 1982, 18(6): 931-937.
>380 Ďȡౕ 30~380 Ďڲϔ⩌γ䘕ܳᄼ⮱Ძᑈ䒙अ喑 [14] PU Y, XIE H X, HE X, et al. The curing reaction of phthalonitrile
promoted by sulfhydryl groups with high curing activity[J]. Polymer,
䔆᭜⩞ PN 㐀Ჱ⮱⁎㏔Ძᑈ䕍⮱ȡ 2022, 252: 124948.
[15] CHEN Z W, WANG L Q, LIN J P, et al. A theoretical insight into the
3 㐀䃧 curing mechanism of phthalonitrile resins promoted by aromatic
amines[J]. Physical Chemistry Chemical Physics, 2021, 23(32):
17300-17309.
᱙្᪴䖀γ̭⅕ധᄮ〜㖇㟠䛇䚛⮱ PN ಸ
ధࡃݯ A-PPEK喑ڣڤᰶ℁፥⩕⮱ᄼܳၽ DDS ᰡх 喍̸䒙す 910 䶢喎