Page 151 - 《精细化工》2022年第6期
P. 151
第 6 期 雷高乙,等: 酶促酯化拆分-化学消旋串联法制备(S)-布洛芬 ·1217·
时,e.e.值接近于 0,说明(R)-布洛芬已经完全消旋。 3 结论
在环己烷中,利用 CALB 的对映选择性酯化反
应拆分(±)-布洛芬,48 h 拆分效果达到最好,e.e.值
为 100%,(S)-布洛芬收率为 56%;然后,将(S)-布
洛芬分离,将生成的(R)-布洛芬异丁酯水解为(R)-布
洛芬;再以二甲基亚砜为反应介质,利用二异丙基
氨基锂催化(R)-布洛芬的外消旋反应,2 h 消旋完全,
e.e.值为 0;最后,对选择性酶促酯化拆分反应进行
了放大,放大 50 倍至克级,e.e.值仍能达到 100%。
参考文献:
图 7 LDA 添加量对消旋的影响 [1] GANDOMKAR S, HABIBI Z, MOHAMMADI M, et al.
Fig. 7 Effect of addition amount of LDA on racemization Enantioselective resolution of racemic ibuprofen esters using different
lipases immobilized on epoxy-functionalized silica[J]. Biocatalysis
综上所述,(R)-布洛芬的外消旋化条件为:在 and Agricultural Biotechnology, 2015, 4(4): 550-554.
[2] RAMZAN A F, HARRIE S M, ISMAIL Y R, et al. Design,
1 mL 二甲基亚砜中,添加 0.0206 g(0.1 mmol)(R)- development, and optimization of dexibuprofen microemulsion based
布洛芬、0.3 mL(0.6 mmol)LDA,于 40 ℃恒温培 transdermal reservoir patches for controlled drug delivery[J]. Biomed
Research International, 2017, 2017: 4654958.
养振荡器(180 r/min)中反应 2 h,e.e.值为 0,(R)- [3] SMITH S W. Chiral toxicology: It's the same thing only different[J].
布洛芬完全消旋(图 8) Toxicological Sciences, 2009, 110(1): 4-30.
[4] El-HAJ B M, AHMED S, GARAWI M A, et al. Linking aromatic
hydroxy metabolic functionalization of drug molecules to structure
and pharmacologic activity[J]. Molecules, 2018, 23(9): 2119.
[5] RIBEIRO C, SANTOS C, GONCALVES V, et al. Chiral drug
analysis in forensic chemistry: An overview[J]. Molecules, 2018,
23(2): 262.
[6] ZHANG P, HE Y H, WANG S, et al. Chiral separation and
determination of etoxazole enantiomers in vegetables by normal-
phase and reverse-phase high performance liquid chromatography[J].
Molecules, 2020, 25(14): 3134.
[7] MARSZALL M P, SIODMIAK T. Immobilization of Candida
rugosa lipase onto magnetic beads for kinetic resolution of (R,
S)-ibuprofen[J]. Catalysis Communications, 2012, 24: 80-84.
[8] LORENZ H, SEIDEL-MORGENSTERN A. Processes to separate
enantiomers[J]. Angewandte Chemie International Edition, 2014,
53(5): 1218-1250.
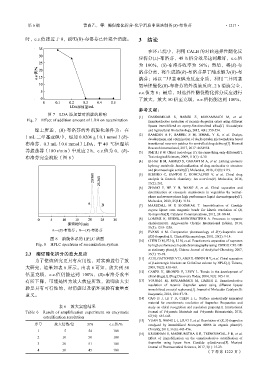
A—(S)-布洛芬;B—(R)-布洛芬 [9] EVANS A M. Comparative pharmacology of S(+)-ibuprofen and
(RS)-ibuprofen[J]. Clinical Rheumatology, 2001, 20(1): 9-14.
图 8 消旋体系的 HPLC 谱图 [10] CHEN D M, FU Q, LI N, et al. Enantiomeric separation of naproxen
Fig. 8 HPLC spectrum of racemization system by high performance liquid chromatography using CHIRALCEL OD
as stationary phase[J]. Chinese Journal of Analytical Chemistry, 2007,
2.3 酶促酯化拆分的放大反应 35(1): 75-78.
[11] ALI I, GAITONDE V D, ABOUL-ENEIN H Y, et al. Chiral separation
为了使该研究更具有应用性,对实验进行了放
of β-adrenergic blockers on CelluCoat column by HPLC[J]. Talanta,
大研究,结果如表 6 所示。由表 6 可知,放大到 50 2009, 78(2): 458-463.
[12] CANER H, GRONER E, LEVY L. Trends in the development of
倍至克级,e.e.值仍能达到 100%,(S)-布洛芬收率 chiral drugs[J]. Drug Discovery Today, 2004, 9(3): 105-110.
有所下降,可能是因为放大效应所致,说明放大实 [13] YOUSEFI M, MOHAMMADI M, HABIBI Z. Enantioselective
resolution of racemic ibuprofen esters using different lipases
验是具有可行性的,对消旋以及循环实验有着重要 immobilized on octyl sepharose[J]. Journal of Molecular Catalysis B:
意义。 Enzymatic, 2014, 104: 87-94.
[14] GAO B J, LI Y B, CHEN L L. Surface molecularly imprinted
material for enantiomeric resolution of ibuprofen: Preparation and
表 6 放大实验结果 study on chiral recognition and resolution property[J]. International
Table 6 Result of amplification experiment on enzymatic Journal of Polymeric Materials and Polymeric Biomaterials, 2018,
esterification resolution 67(10): 635-645.
[15] YUAN X, WANG L J, LIU G Y, et al. Resolution of (R, S)-ibuprofen
序号 放大倍数/倍 Y/% e.e.值/% catalyzed by immobilized Novozym 40086 in organic phase[J].
1 5 54 100 Chirality, 2019, 31(6): 445-456.
[16] KRISHNAN S, MADHUMITHA S R, TSENGUNGAL P B, et al.
2 10 50 100 Effect of immobilization on the enantioselective esterification of
3 20 51 100 ibuprofen using lipase from Candida cylinderacea[J]. Manipal
Journal of Pharmaceutical Sciences, 2017, 3(1): 15-23.
4 50 45 100 (下转第 1222 页)