Page 52 - 201807
P. 52
·1120· 精细化工 FINE CHEMICALS 第 35 卷
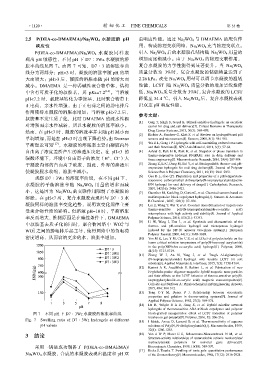
2.5 P(DEA-co-DMAEMA)/Na 2 WO 4 水凝胶的 pH 重响应性能。通过 Na 2 WO 4 与 DMAEMA 的配位作
响应性 用,构成物理交联网络,Na 2 WO 4 充当物理交联点。
P(DEA-co-DMAEMA)/Na 2 WO 4 水凝胶 同样表 引入 Na 2 WO 4 后的水凝胶孔结构随 Na 2 WO 4 用量的
现出 pH 敏感性,不同 pH 下 D7∶3Wz 水凝胶的溶 增加而逐渐减小。由于 Na 2 WO 4 的物理交联作用,
胀率曲线见图 7。由图 7 可知,D7∶3 的溶胀率曲 复合水凝胶的力学性能得到显著提升。当 Na 2 WO 4
线分为两部分:pH<3 时,凝胶的溶胀率随 pH 的增 质量分数为 3%时,复合水凝胶的储能模量达到了
大而增大;pH>3 后,凝胶的溶胀率随 pH 的增大而 2.26 kPa。改变 Na 2 WO 4 用量可以调节水凝胶的温敏
减小。DMAEMA 是一种弱碱性聚合物单体,结构 性能,LCST 随 Na 2 WO 4 质量分数的增加呈线性降
中含有可质子化的叔胺基,其 pKa=7.2 [16] 。当溶液 低,Na 2 WO 4 质量分数为 3%时,复合水凝胶的 LCST
pH<7.2 时,叔胺基转化为季铵基,此时聚合物带上 降低至 34.4 ℃。引入 Na 2 WO 4 后,复合水凝胶表现
正电荷,亲水性增强,由于正电荷之间的静电排斥 出双重 pH 响应性能。
作用使得水凝胶的溶胀率增加;当溶液 pH>7.2 后,
参考文献:
叔胺基不发生质子化,此时 DMAEMA 的疏水性相
[1] Garg T, Singh S, Goyal K. Stimuli-sensitive hydrogels: an excellent
对增强而亲水性减弱,所以水凝胶的溶胀率减小。 carrier for drug and cell delivery[J]. Critical Reviews in Therapeutic
然而,在 pH<7 时,凝胶的溶胀率并未随 pH 减小而 Drug Carrier Systems, 2013, 30(5): 369-409.
[2] Richter A, Paschew G, Klatt S, et al. Review on hydrogel-based pH
单调增加,而是在 pH<3 时出现下降趋势。由 Donnan sensors and microsensors[J]. Sensors, 2008, 8(1): 561-581.
平衡理论可知 [17] ,水凝胶的溶胀率主要由凝胶内外 [3] Wu Z L, Gong J P. Hydrogels with self-assembling ordered structures
and their functions[J]. NPG Asia Material, 2011, 3(3): 57-64.
自由离子浓度差所产生的渗透压决定。在 pH<3 的 [4] Ashraf S, Park H K, Park H, et al. Snapshot of phase transition in
+
–
强酸环境下,环境中自由离子的浓度(H 、Cl )大 thermo-responsive hydrogel PNIPAM: role in drug delivery and
tissue engineering[J]. Macromolecular Research, 2016, 24(4): 297-304.
于凝胶内部的自由离子浓度,因此,外界的渗透压 [5] Zhang Z, Li C, Deng M, Bai Y, et al. Biodegradable thermo- and pH-
responsive hydrogels for oral drug delivery[J]. Journal of Polymer
使凝胶脱水收缩,溶胀率减小。 Science Part A Polymer Chemistry, 2011, 49(13): 2941-2951.
观察 D7∶3Wz 的溶胀率曲线,在不同 pH 下, [6] Guo B L, Gao QY. Preparation and properties of a pH/temperature-
responsive carboxymethyl chitosan/poly(N-isopropylacrylamide)semi-
水凝胶的平衡溶胀率随 Na 2 WO 4 用量的增多而减 IPN hydrogel for oral delivery of drugs[J]. Carbohydrate Research,
2007, 342(16): 2416-2422.
小,这是因为 Na 2 WO 4 的交联作用限制了水凝胶的 [7] Guenther M, Kuckling D, CortenC, et al. Chemical sensors based on
溶胀。在 pH<7 时,复合水凝胶表现出与 D7∶3 水 multiresponsive block copolymer hydrogels[J]. Sensors & Actuators
B Chemical , 2007, 126(1): 97-106.
凝胶同样的溶胀率变化趋势,说明该变化规律主要 [8] Lai E, Wang Y, Wei Y, et al. Covalent immobilization of trypsin onto
受聚合物性质的影响。但溶液 pH=10 时,平衡溶胀 thermo-sensitive poly(N-isopropylacrylamide-co-acrylic acid)
microspheres with high activity and stability[J]. Journal of Applied
率反而增大,推测原因是在碱性条件下,DMAEMA Polymer Science, 2016, 133(21): 43343.
2–
中叔胺基去质子化的同时,聚合物网络中 WO 4 与 [9] Li W, Wang J, Zou L, et al. Synthesis and characteristic of the
thermo- and pH-sensitive hydrogel and microporous hydrogel
2–
WO 4 之间的静电排斥起主导,使得网络中的负电荷 induced by the NP-10 aqueous two-phase system[J]. European
Polymer Journal, 2008, 44(11): 3688-3699.
密度增高,从而容纳更多的水,溶胀率增加。 [10] Yoo M K, Lee Y M, Cho C S, et al. Effect of polyelectrolyte on the
lower critical solution temperature of poly(N-isopropyl acrylamide)
in the poly(NIPAAm-co-acrylic acid) hydrogel[J]. Polymer, 2000,
41(15): 5713-5719.
[11] Zheng W J, An N, Yang J, et al. Tough Al-alginate/poly
(N-isopropylacrylamide) hydrogel with tunable LCST for soft
robotics[J]. Applied Materials & Interfaces, 2015, 7(3): 1758-1764.
[12] Karimi A R, Azadikhah F, Rahimi L, et al. Fabrication of new
Fe-phthalocyanine oligomer-magnetite hybrid magnetic nano particles
and their effects on the LCST behavior of thermo-sensitive poly(N-
isopropylacrylamide-co-acrylic acid) magnetic nanocomposites[J].
Colloids and Surfaces A: Physicochemical and Engineering Aspects,
2015, 484: 304-312.
[13] Tung C-Y M, Dynes P J. Relationship between viscoelastic
properties and gelation in thermo-setting systems[J]. Journal of
Applied Polymer Science, 1982, 27(2): 569-574.
[14] Hu B, Wright R A E, Jiang S, et al. Hybrid micellar network
hydrogels of thermosensitive ABA triblock copolymer and polymer
图 7 不同 pH 下 D7∶3Wz 水凝胶的溶胀率曲线 brush-grafted nanoparticles: effect of LCST transition of polymer
brushes on gel property[J]. Polymer, 2016, 82: 206-216.
Fig. 7 Swelling ratio of D7∶3Wz hydrogels at different [15] I Idziak, Avoce D, Lessard D, et al. Thermosensitivity of aqueous
pH values solutions of Poly(N, N-diethylacrylamide)[J]. Macromolecules, 1999,
32(4): 1260-1263.
3 结论 [16] Van d W P, Moret E E, Schuurmans-Nieuwenbroek N M, et al.
Structure-activity relationships of water-soluble cationic methacrylate/
methacrylamide polymers for nonviral gene delivery[J].
采用一锅法成功制备了 P(DEA-co-DMAEMA)/ Bioconjugate Chemistry, 1999, 10(10): 589-597.
[17] Ricka J, Tanaka T. Swelling of ionic gels: quantitative performance
Na 2 WO 4 水凝胶,合成的水凝胶表现出温度和 pH 双 of the Donnan theory[J]. Macromolecules, 1984, 17(12): 2916-2921.