Page 122 - 《精细化工》2023年第4期
P. 122
g808g ㏳ࡃጒ FINE CHEMICALS す 40 ࢤ
UCNPs ᰶۼᑞ喠ฺౕव MnO 2 ऻ喑UPM ⮱ࣾᄱም 䓴⼸͚ສ⅔㏳㣹㏳㘋ڲϔ⩌⮱⅔ࡃ✳ࣾजҬ㏱㏴͚
Ѻ㒛᱗ࣾ⩌⼨ߕ喑⩞λ MnO 2 ⮱॥ᩣፓ̻ UCNPs ⮱ H 2 O 2 ⅡᎠᔘ䕌ࡴ倅㜠℘ᦖᅁ㏔⊀Ꮣ喑́㏳㣹㏳㘋䉕
ࣾᄱ䅞ࣾ⩌䛺व喑䲋ࣾٶܳ MnO 2 ᄦ UCNPs ⮱ࣾ ռᑞ䚥ᕔ [30-31] 喑̷䔝㐀ᘼঠⱭ UPM ڤᰶ䕇䓴יࡃ
ᄱٶ⮱॥ᩣ҉⩕ᄩ㜡 UPM-x ⮱ࣾٶᑧᏓ䮼Ɑ MnO 2 ܳ㼐ڲ⎽ᕔ H 2 O 2 倅㏳㣹ᓛ⣜ධ͚⏣⅔䛼⮱㘪߈喑
䉌䒪䛼⮱๔䔽⌽㶝ۼ喑अࡃ䊸̻߬ ZHANG ぶ [20] ϻ㔹ᰶ᱈倅 PDT ោ㣹⮱᩵⢴ȡ
ᄦٶ᩼ݯ UCNPs@TiO 2 @MnO 2 ⮱ⵁ⾣㐀ㆨѩ喑䔆
॥ᩣ҉⩕᭜⓭≨ MnO 2 ϔ⩌ ROS ⮱ݺȡ
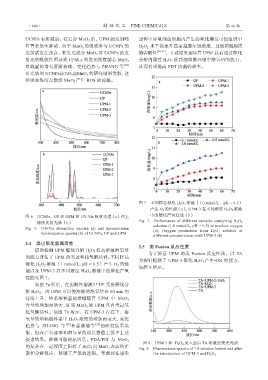
ఫ 7 ̺हᵤ৮יࡃ H 2 O 2 ⏣⋟喍1.0 mmol/LȠpH = 6.5喎
ϔ⩌ O 2 ⮱ᕔ㘪喍a喎喠UPM-3 ౕ̺ह⊀Ꮣ H 2 O 2 ⏣⋟
ఫ 6 UCNPsȠUP হ UPM ⮱ UV-Vis ॥ᩣٶ䅞喍a喎হ̷ ͚⮱יࡃϔ⅔ᕔ㘪喍b喎
䒙ᢏࣾᄱٶ䅞喍b喎 Fig. 7 Performance of different samples catalyzing H 2 O 2
Fig. 6 UV-Vis absorption spectra (a) and upconversion solution (1.0 mmol/L, pH = 6.5) to produce oxygen
(a); Oxygen production from H 2 O 2 solution at
luminescence spectra (b) of UCNPs, UP and UPM different concentrations with UPM-3 (b)
2.4 ㆨ䓴⅔ࡃ⅏䚣≨ᕔ
2.5 ㆨ Fenton ࣺᏁᕔ䉕
Ռߖᷭ≸ UPM יࡃܳ㼐 H 2 O 2 倅⏣⋟⏣⅔䛼
ͧγ侹䃮 UPM ⮱ㆨ Fenton ࣺᏁᕔ䉕喑В TA
⮱㘪߈䃱ѝγ UPM ⮱ㆨ䓴⅔ࡃ⅏䚣≨ᕔȡ̺हᵤ৮
ͧᣏ䦵ᷭ≸γ UPM-3 יࡃ H 2 O 2 ϔ⩌•OH ⮱㘪߈喑
יࡃ H 2 O 2 ⏣⋟喍1 mmol/L, pH = 6.5喎ϔ⩌ O 2 ⮱ᕔ
ຯఫ 8 ȡ
㘪Вࣷ UPM-3 ౕ̺ह⊀Ꮣ H 2 O 2 ⏣⋟͚⮱יࡃϔ⅔
ᕔ㘪㻮ఫ 7ȡ
ຯఫ 7a 喑ౕᑞ䚥ᕔ⏣⋟͚ UP ᬍ∂יࡃܳ
㼐 H 2 O 2 喑㔹 UPM जВҬ⏣⋟⮱⏣⅔䛼ౕ 60 min ڲ
ᠮ㐚̷ࡴ喑Ҁ㈨⏣⅔䛼⮱፲䮼Ɑ UPM ͚ MnO 2
क़䛼⮱ߍ㔹๔喑䃮ᬻ MnO 2 Ҭ UPM ڤᰶㆨ䓴⅔
ࡃ⅏䚣≨ᕔȡຯఫ 7b 喑ౕ UPM-3 ႅ̸ౕ喑⏣
⅔䛼⮱፲䮼Ҁ㈨͚ H 2 O 2 ⊀Ꮣ⮱ߍ㔹अ๔喑अࡃ
䊸̻߬ ZHANG ぶ [20] হᰦ⑴ẍぶ [29] ⮱ⵁ⾣㐀ㆨ
ѩ喑ѳౕϔ⅔䕌⢴হ⏣⅔䛼⮱䪬թ̷ѻλ̷䔝
្䖀㐀ȡᣕ≸ज㘪⮱࣌᭜喑PDA/PEI ̻ MnO 2
ఫ 8 UPM-3 হ H 2 O 2 ߍڒݺऻ TA ⏣⋟⮱㢔ٶٶ䅞
⮱ฺवౕ̭Ⴧ⼸Ꮣ̷䭨ⶺγ H 2 O 2 ा MnO 2 㶕䲏⮱ព Fig. 8 Fluorescence spectra of TA solution before and after
᪐হܳ㼐ࣺᏁ喑ᐣ㑀γϔ⅔⮱䔈⼸ȡ㔰㭾ݝౕᙌᴀ the introduction of UPM-3 and H 2 O 2