Page 141 - 201811
P. 141
第 11 期 周燕强,等: Pt/MoC 的制备及其在电解水析氢反应中的催化性能 ·1927·
相态在碳化合成过程中通过形貌及体相上的拓扑变
换过程保持了样品整体上的片层结构,暴露出更多
的活性位点,相比于 β-Mo 2 C,α-MoC 表现出更大的
比表面积及更好的 HER 催化活性,更加有利于电解
水析氢反应的进行。
(2)XPS 结果显示,随着 Pt 负载量的增加,Pt
与碳化钼载体的相互作用也随之增强,1.6Pt/Mo x C y
的高 HER 催 化活性及 稳定性的 原因归结 于
α-MoC 1–x 的高表面积及 Pt 与 MoC 的强相互作用。
1.6Pt/Mo x C y 在酸性电解质中过电势 η onset =108 mV,
塔菲尔斜率 b=74 mV/dec,阻抗为 18.77 Ω。
(3)对比价格昂贵的商业 20%Pt/C(20%为 Pt
的质量分数)催化剂,自制的催化剂可以大幅减少 Pt
金属的用量,成本大约只有商业催化剂的 10%。
参考文献:
[1] Steele B C, Heinzel A. Materials for fuel-cell technologies[J]. Nature,
2001, 414(6861): 345-352.
[2] Kreuter W, Hofmannz H. The important energy transformer in a
world of sustainable energy[J]. International Journal of Hydrogen
Energy, 1996, 23(8): 661-666.
[3] Debe M K. Electrocatalyst approaches and challenges for automotive
fuel cells[J]. Nature, 2012, 486(7401): 43-51.
[4] Walter M G, Warren E L, McKone J R, et al. Solar water splitting
cells[J]. Chemical Reviews, 2010, 110(11): 6446-6473.
[5] Bartak D E, Kazee B, Shimazu K, et al. Electrodeposition and
characterization of platinum microparticles in poly(4-vinylpyridine)
film electrodes[J]. Analytical Chemistry, 1986, 58(13):2756-2761.
[6] Millet P, Andolfatto F, Durand R. Design and performance of a solid
polymer electrolyte water electrolyzer [J]. International Journal of
Hydrogen Energy, 1996, 21(2): 87-93.
[7] Levy R B, Boudan M. Platinum-like behavior of tungsten carbide in
surface catalysis[J]. Science,1973, 181(4099): 547-549.
[8] de Novion C H, Landesman J P. Order and disorder in transition
metal carbides and nitrides: experimental and theoretical aspects[J].
Pure and Applied Chemistry, 1985, 57(10): 1391-1402.
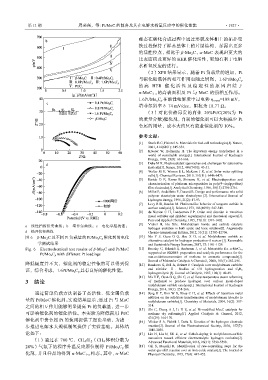
a—线性扫描伏安曲线;b—塔菲尔曲线;c—电化学阻抗谱; [9] Vrubel H, Hu Xile. Molybdenum boride and carbide catalyze
hydrogen evolution in both acidic and basic solutions[J]. Angewandte
d—循环伏安曲线 Chemie-International Edition, 2012, 51(51): 12703-12706.
图 6 β-Mo 2 C 及不同 Pt 负载量的 Pt/Mo x C y 催化剂的电化 [10] Ma Y F, Guan G Q, Hao X G, et al. Molybdenum carbide as
alternative catalyst for hydrogen production-A review [J]. Renewable
学测试结果 and Sustainable Energy Reviews, 2017, 75: 1101-1129.
Fig. 6 Electrochemical test results of β-Mo 2 C and Pt/MoC [11] Bouchy C, Schmidt I, Anderson J, et al. Metastable fcc α-MoC 1-x
Pt/Mo x C y with different Pt loadings supported on HZSM5: preparation and catalytic performance for the
non-oxidativeconversion of methane to aromatic compounds[J].
Journal of Molecular Catalysis A Chemical , 2000, 163(1): 283-296.
降低幅度并不大,催化剂的稳定性依然可以得到保 [12] Ranhotra G, Bell A, Reimer J. Catalysis over molybdenum carbides
证,综合考虑,1.6Pt/Mo xC y 具有良好的催化性能。 and nitrides: Ⅱ . Studies of CO hydrogenation and C 2H 6
hydrogenolysis [J]. Journal of Catalysis, 1987, 108(1): 40-49.
[13] Ma Y F, Guan G Q, Shi C, et al. Low-temperature steam reforming
3 结论 of methanol to produce hydrogen over various metal-doped
molybdenum carbide catalysts[J]. International Journal of Hydrogen
Energy, 2014, 39(1): 258-266.
通过简单负载方法制备了高活性、低金属负载 [14] Jung K T, Kim W B, Rhee C H, et al. Effects of transition metal
addition on the solidstate transformation of molybdenum trioxide to
量的 Pt/MoC 催化剂。实验结果显示,通过 Pt 与 MoC molybdenum carbides[J]. Chemistry of Materials, 2004, 16(2): 307-
之间的相互作用能够明显提高 Pt 的负载量,进一步 314.
[15] Shi C, Zhang A J, Li X S, et al. Ni-modified Mo 2C catalysts for
可影响催化剂的催化活性。本实验为降低商用 Pt/C methane dry reforming[J]. Applied Catalysis A: General, 2012,
431(29): 164-170.
催化剂中贵金属 Pt 的使用提供了理论基础,为进一 [16] Vilekar S A, Fishtik I, Datta R. Kinetics of the hydrogen electrode
步推进电解水大规模制氢提供了实验基础。具体结 reaction[J]. Journal of the Electrochemical Society, 2010, 157(7):
1040-1050.
论如下: [17] Lin H, Liu N, Shi Z, et al. Cobalt-doping in molybdenum-carbide
nanowires toward efficient electrocatalytic hydrogen evolution[J].
(1)通过在 700 ℃、CH 4 /H 2 (CH 4 体积分数为 Advanced Functional Materials, 2016, 26(31): 5590-5598.
20%)气氛下的程序升温反应原位制得 Pt/Mo x C y 催 [18] Oki S, Mezalki R. Identification of rate-controlling steps for the
water-gas shift reaction over an iron oxide catalyst[J]. The Journal of
化剂,并且样品均得到 α-MoC 1–x 相态。其中,α-MoC Physical Chemistry, 1973, 77(4): 447-452.