Page 217 - 《精细化工》2022年第7期
P. 217
第 7 期 唐裕才,等: 可见光促进荧光素催化的氧化偶联反应合成苯并咪唑[2,1-a]异喹啉-6(5H)-酮衍生物·1503·
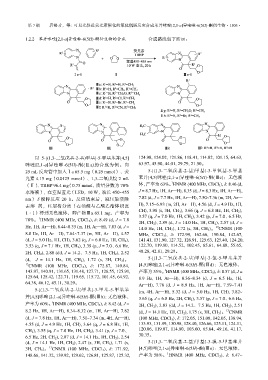
1.2.2 苯并咪唑[2,1-a]异喹啉-6(5H)-酮衍生物的合成 合成路线如下所示:
以 5-[(1,3-二氧戊基-2-基)甲基]-5-甲基苯并[4,5] 124.98, 124.02, 121.86, 118.41, 114.82, 101.15, 64.63,
咪唑[2,1-a]异喹啉-6(5H)-酮(Ⅲa)的合成为例。向 63.97, 45.90, 44.01, 29.79, 21.50。
25 mL 反应管中加入Ⅰa 65.5 mg(0.25 mmol)、荧 5-[(1,3-二氧戊基-2-基)甲基]-3-甲氧基-5-甲基
光素 4.15 mg(0.0125 mmol)、1,3-二氧戊烷 2 mL 苯并[4,5]咪唑[2,1-a]异喹啉-6(5H)-酮(Ⅲc):无色液
1
(Ⅱ)、TBHP 96.4 mg(0.75 mmol,质量分数为 70% 体,产率为 63%。HNMR (400 MHz, CDCl 3), δ: 8.46 (d,
水溶液),在室温蓝光(LED,10 W,波长 450~455 J = 8.7 Hz, 1H, Ar—H), 8.35 (d, J = 8.3 Hz, 1H, Ar—H),
nm)下搅拌反应 20 h。反应结束后,减压旋蒸除 7.82 (d, J = 7.7 Hz, 1H, Ar—H), 7.50~7.36 (m, 2H, Ar—
去溶 剂,柱层析分离(石油醚与乙酸乙酯体积比 H), 7.15~6.93 (m, 2H, Ar—H), 4.56 (d, J = 4.9 Hz, 1H,
CH), 3.98 (s, 3H, CH 3 ), 3.66 (q, J = 6.8 Hz, 1H, CH 2 ),
1∶1)得到无色液体,即产物Ⅲa 65.1 mg,产率为
1
78%。 HNMR (400 MHz, CDCl 3 ), δ: 8.49 (d, J = 7.8 3.57 (q, J = 7.0 Hz, 1H, CH 2 ), 3.42 (p, J = 7.0、6.5 Hz,
2H, CH 2 ), 2.89 (d, J = 14.0 Hz, 1H, CH 2 ), 2.57 (d, J =
Hz, 1H, Ar—H), 8.44~8.33 (m, 1H, Ar—H), 7.83 (d, J = 14.0 Hz, 1H, CH 2 ), 1.72 (s, 3H, CH 3 )。 CNMR (100
13
8.8 Hz, 1H, Ar—H), 7.64~7.37 (m, 5H, Ar—H), 4.57 MHz, CDCl 3 ), δ: 172.99, 162.66, 150.84, 142.67,
(d, J = 5.0 Hz, 1H, CH), 3.62 (q, J = 6.8 Hz, 1H, CH 2 ), 141.41, 131.90, 127.72, 126.91, 125.63, 125.40, 124.20,
3.53 (q, J = 7.1 Hz, 1H, CH 2 ), 3.39 (p, J = 7.0、6.6 Hz, 122.70, 119.80, 114.52, 102.45, 65.61, 64.88, 55.65,
2H, CH 2 ), 2.88 (dd, J = 14.2、7.5 Hz, 1H, CH 2 ), 2.52 48.28, 42.81, 29.29。
(d, J = 14.1 Hz, 1H, CH 2 ), 1.72 (s, 3H, CH 3 )。 5-[(1,3-二氧戊基-2-基)甲基]-3-氯-5-甲基苯并
13 CNMR (100 MHz, CDCl 3 ), δ: 172.87, 149.84, [4,5]咪唑[2,1-a]异喹啉-6(5H)-酮(Ⅲd):无色液体,
1
143.97, 140.91, 131.65, 131.48, 127.71, 126.55, 125.91, 产率为 55%。HNMR (400 MHz, CDCl 3), δ: 8.57 (d, J =
125.64, 125.42, 122.71, 119.65, 115.72, 101.45, 64.92, 8.9 Hz, 1H, Ar—H), 8.36~8.34 (d, J = 6.5 Hz, 1H,
64.38, 46.12, 45.11, 30.29。
Ar—H), 7.76 (d, J = 8.9 Hz, 1H, Ar—H), 7.59~7.41
5-[(1,3-二氧戊基-2-基)甲基]-3-甲基-5-甲基苯
(m, 4H, Ar—H), 5.32 (d, J = 5.0 Hz, 1H, CH), 3.82~
并[4,5]咪唑[2,1-a]异喹啉-6(5H)-酮(Ⅲb):无色液体, 3.65 (q, J = 6.8 Hz, 2H, CH 2 ), 3.57 (p, J = 7.0、6.6 Hz,
1
产率为 60%。HNMR (400 MHz, CDCl 3), δ: 8.42 (d, J = 2H, CH 2 ), 2.83 (dd, J = 14.2、7.5 Hz, 1H, CH 2 ), 2.51
8.2 Hz, 1H, Ar—H), 8.34~8.32 (m, 1H, Ar—H), 7.82 (d, J = 14.1 Hz, 1H, CH 2), 1.75 (s, 3H, CH 3 )。 CNMR
13
(d, J = 7.8 Hz, 1H, Ar—H), 7.51~7.34 (m, 4H, Ar—H), (100 MHz, CDCl 3 ), δ: 172.05, 151.08, 142.05, 138.94,
4.55 (d, J = 4.9 Hz, 1H, CH), 3.64 (q, J = 6.8 Hz, 1H, 133.93, 131.99, 130.98, 128.40, 126.66, 125.11, 124.11,
CH 2 ), 3.55 (q, J = 7.0 Hz, 1H, CH 2 ), 3.41 (p, J = 7.0、 120.06, 119.07, 114.80, 103.60, 65.64, 49.16, 41.17,
6.5 Hz, 2H, CH 2 ), 2.87 (d, J = 14.1 Hz, 1H, CH 2 ), 2.54 30.35。
(d, J = 14.1 Hz, 1H, CH 2 ), 2.47 (s, 3H, CH 3 ), 1.71 (s, 5-[(1,3-二氧戊基-2-基)甲基]-3-溴-5-甲基苯并
13
3H, CH 3 )。 CNMR (100 MHz, CDCl 3 ), δ: 171.92, [4,5]咪唑[2,1-a]异喹啉-6(5H)-酮(Ⅲe):无色液体,
1
148.66, 141.32, 139.92, 129.62, 126.81, 125.87, 125.32, 产率为 58%。 HNMR (400 MHz, CDCl 3 ), δ: 8.47~