Page 157 - 《精细化工》2023年第3期
P. 157
第 3 期 尹金佩,等: Mn 3 O 4 微观结构对固相合成类单晶锰酸锂的影响 ·613·
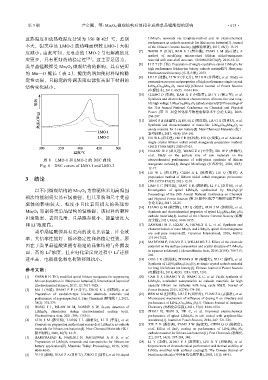
放热温度和放热峰温度分别为 350 和 425 ℃,差别 LiMn 2O 4 nanorods via template-method and its electrochemical
performance as cathode materials for lithium ion batteries[J]. Journal
不大,但类单晶 LMO-2 放热峰面积较 LMO-1 大幅 of the Chinese Ceramic Society (硅酸盐学报), 2017, 45(1): 15-19.
度减小。由此可知,充电态的 LMO-2 与电解液的反 [7] WANG T (汪涛), FAN S J (樊少娟), YANG L M (杨立铭). A
method of modifying micron-sized lithium nickel-manganate
应更少,具有更好的热稳定性 [24] 。这主要是因为, material with core-shell structure: CN106025267A[P]. 2016-10-12.
[8] LI Z T (李子涛). Preparation of single-crystalline spinel LiMn 2O 4 for
类单晶锰酸锂受 Mn 3 O 4 微观结构的影响,具有更短 high performance lithium-ion battery cathode material[D]. Shenyang:
的 Mn—O 键长(表 2),键能的增加使材料结构稳 Northeastern University (东北大学), 2013.
[9] LIU P (刘攀), LI W S (李文升), XU G F (许国峰), et al. Study on
定性更强,且稳定的外露表面也能使高温下材料的 preparation process and properties of high performance single crystal
结构变化减小。 LiNi 0.5Co 0.2Mn 0.3O 2 material[J].Chinese Journal of Power Sources
(电源技术), 2019, 43(7): 1104-1106.
[10] CHENG D (程迪), TIAN X Y (田新勇), XU Y J (徐云军), et al.
Synthesis and electrochemical characteristics of mono-like and long-
life high voltage LiNi 0.5Co 0.2Mn 0.3O 2 cathode material[C]// Proceedings of
the 31st Annual National Conference on Chemical and Physical
Power (第 31 届全国化学与物理电源学术年会论文集), 2015:
204-207.
[11] TANG S H (唐盛贺), ZHOU H Z (周汉章), LIU G H (刘更好), et al.
Synthesis and characterization of mono-like LiNi 0.6Co 0.2Mn 0.2O 2 as
anode material for Li-ion battery[J]. New Chemical Materials (化工
新型材料), 2017, 45(4): 139-141.
[12] HU W L (胡文理), HE F R (何凤荣), HU Q (胡骐), et al. A kind of
single crystal lithium nickel cobalt manganate preparation method:
CN111370683A[P]. 2020-07-03.
[13] HUANG W J (黄文进), WANG Z J (王泽杰), DU W P (杜婉萍),
et al. Study on the particle size of raw materials on the
图 8 LMO-1 和 LMO-2 的 DSC 曲线 electrochemical performance of solid-phase synthesis of lithium
Fig. 8 DSC curves of LMO-1 and LMO-2 manganate cathode[J]. Jiangxi Metallurgy (江西冶金), 2020, 40(3):
12-17.
[14] HU W L (胡文理), CHEN H L (陈海轮), HU Q (胡骐). A
3 结论 preparation method of lithium nickel cobalt manganate precursors:
CN113735187A[P]. 2021-12-03.
[15] HAN Y C (韩要丛), TANG Y B (唐跃波), LI P L (李普良), et al.
以不同微观结构的 Mn 3 O 4 为前驱体采用高温固 Investigation of spinel LiMn 2O 4 synthesized by Mn 3O 4[C]//
Proceedings of the 29th Annual National Conference on Chemical
相法均能制得尖晶石锰酸锂,但其形貌和尺寸受前 and Physical Power Sources (第 29 届全国化学与物理电源学术年
驱体的影响较大,粒度小且比表面积大的类球形 会论文集), 2011: 22-25.
[16] LIANG Q M (梁其梅), LIU Q (刘清), GUO J M (郭俊明), et al.
Mn 3 O 4 易制得类单晶结构的锰酸锂,该材料的颗粒 Synthesis and electrochemical properties of spinel Li 1.02Ni 0.05Mn 1.93O 4
团聚致密、表面光滑,且晶胞参数小、能量密度大 cathode materials[J]. Journal of the Chinese Ceramic Society (硅酸
盐学报), 2021, 49(6): 1048-1055.
+
和 Li 浓度高。 [17] ZAWRAH M F, EZZAT A, FADALY E L, et al. Synthesis and
characterization of nano Mn 3O 4 and LiMn 2O 4 spinel from manganese
类单晶锰酸锂具有更高的放电比容量、库仑效 ore and pure materials[J]. Ceramics International, 2020, 46(11):
率,大倍率性能好、循环稳定性和热稳定性强。原 17514-17522.
[18] MARCHNI F, CALVO E J, WILLIAMS F J. Effect of the electrode
因在于类单晶锰酸锂拥有稳定的晶体结构与外露表 potential on the surface composition and crystal structure of LiMn 2O 4
+
+
面、高的 Li 浓度,且在电化学反应过程中 Li 迁移 in aqueous solutions[J]. Electrochimica Acta, 2018, 2(108): 706-713,
269.
速率高、电极极化和电荷转移阻抗小。 [19] GUO J K (郭进康), ZHONG S W (钟盛文), XU C (徐唱), et al.
Synthesis of Li(Ni 1/3Mn 1/3Co 1/3)O 2 as single crystal cathode material
参考文献: for long life lithium ion battery[J]. Chinese Journal of Power Sources
(电源技术), 2018, 42(9): 1283-1285, 1293.
[1] CHEN H H. TiO 2-modified spinel lithium manganate for suppressing [20] CAN Y J, HUANG Y D, WANG X C, et al. Facile synthesis of
Mn ion dissolution in lithium ion batteries[J]. International Journal of LiMn 2O 4 octahedral nanoparticles as cathode materials for high
Electrochemical Science, 2017, 12: 7817-7828. capacity lithium ion batteries with long cycle life[J]. Journal of
[2] MA J (马婧), WANG F P (王芳平), ZHOU K L (周凯玲), et al. Power Sources, 2015, 1(278): 574-581.
Preparation of sandwich-type biochar electrode materials and [21] REN M M (任明明), LIU Z P (刘泽萍), YUAN Z L (袁振洛), et al.
performance of supercapacitor[J]. Fine Chemicals (精细化工), 2021, Microscopic mechanism of influence of doping F on structure and
38(2): 374-379. performance of LiNi 0.8Co 0.1Mn 0.1O 2[J]. Chinese Journal of Inorganic
[3] WANG J L, ISLAM M M, DONNE S W. In-situ detection of Chemistry (无机化学学报), 2021, 37(6): 1046-1054.
LiMn 2O 4 dissolution during electrochemical cycling by[J]. [22] ZHOU H, WAN S, HE C, et al. Improved electrochemical
Electrochimica Acta, 2021, 386: 138366. performance of spinel LiMn 2O 4 in situ coated with graphene-like
[4] GUO J M (郭佳明), LIANG J L (梁精龙), LI H (李慧), et al. membrane[J]. Journal of Power Sources, 2014, 247: 721-728.
Progress on preparation method and research of LiMn 2O 4 as cathode [23] XIE Z D (谢志迪), YANG J W (杨建文), CHEN Q Q (陈权启),
materials for lithium-ion batteries[J]. New Chemical Materials (化工 et al. Effect of ZnF 2 coating on performance of LiNi 0.5Mn 1.5O 4
新型材料), 2020, 48(7): 43-51. cathode material for lithium-ion batteries[J]. Fine Chemicals (精细化
[5] HARIPRASAD K, NARESH N, NAGESWAR A R B, et al. 工), 2017, 34(3): 297-284, 340.
Preparation of LiMn 2O 4 nanorods and nanoparticles for lithium-ion [24] LI Y (李燕), ZHAO Y J (赵煜娟), LIU X Y (刘欣燕), et al.
battery applications[J]. Materials Today: Proceedings, 2016, 3(10): Improvement of electrochemical performance and thermal stability of
4040-4045. LiNiO 2 modified with surficial coating[J]. The Chinese Journal of
[6] YU G (余刚), XIAO Z A (肖作安), ZHOU Z (周哲), et al. Ni-doped Nonferrous Metals (中国有色金属学报), 2005, 15(1): 88-91.