Page 114 - 201809
P. 114
·1540· 精细化工 FINE CHEMICALS 第 35 卷
催化剂进行 ICP-AES 分析可知,VO@g-C 3 N 4 -T 催 催化活性。剥离成薄片的 g-C 3 N 4 和乙酰丙酮氧钒络
化剂中 V 质量分数仍能达到 9.69%,和新鲜的 9.78% 合可能形成一种特殊的结构,且具有较强的协同效
相比,说明催化剂中钒元素在反应中基本没有溶脱, 应,导致这种催化剂具有合适的带差,对可见光催
这与文献报道基本一致 [11] 。在对反应液进行 ICP-AES 化苯羟基化具有较好的响应。富含氮原子的纳米薄
测试发现,反应液中没有发现钒元素的存在,可能 片石墨化氮化碳表面对于吸收可见光、扩散反应介
是钒化合物和 g-C 3 N 4 具有非常强的结合力所致。结 质也具有重要的作用,促进了苯羟基化中 C—H 的
合套用 5 次后的 SEM(图 4F)表征可知,该催化剂 活化。
套用前、后其形貌没有明显改变,这可能是该催化
参考文献:
剂稳定性较好的重要原因。
[1] Ehrich H, Berndt H, Pohl M-M, et al. Oxidation of benzene to phenol
on supported Pt-VO x and Pd-VO x catalysts [J]. Applied Catalysis A:
General, 2002, 230(1/2): 271-280.
[2] Herron N, Tolman C A. A highly selective zeolite catalyst for
hydrocarbon oxidation. A completely inorganic mimic of the alkane
ω-hydroxylases [J]. Journal of the American Chemical Society,
1987, 109(9): 2837-2839.
[3] Mimoun H, Saussine L, Daire E, et al. Vanadium(Ⅴ) peroxy
complexes. New versatile biomimetic reagents for epoxidation of
olefins and hydroxylation of alkanes and aromatic hydrocarbons [J].
Journal of the American Chemical Society, 1983, 105(10): 3101-3110.
[4] Tani M, Sakamoto T, Mita S, et al. Hydroxylation of benzene to
phenol under air and carbon monoxide catalyzed by
molybdovanadophosphoric acid [J]. Angewandte Chemie International
Edition, 2005, 44(17): 2586-2588.
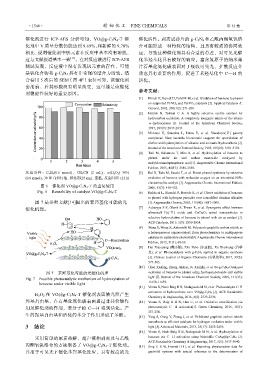
反应 条件 : C 6H 6(0.5 mmol), CH 3CN (2 mL), w(H 2O 2)=30% [5] Bal R, Tada M, Sasaki T, et al. Direct phenol synthesis by selective
(0.6 mmol),30 W 白炽灯泡,催化剂(25 mg),室温,反应时间 (12 h) oxidation of benzene with molecular oxygen on an interstitial-N/Re
cluster/zeolite catalyst [J]. Angewandte Chemie International Edition,
图 6 催化剂 VO@g-C 3 N 4 -T 的重复使用 2006, 45(3): 448-452.
Fig. 6 Reusability of catalyst VO@g-C 3 N 4 -T [6] Balducci L, Bianchi D, Bortolo R, et al. Direct oxidation of benzene
to phenol with hydrogen peroxide over a modified titanium silicalite
图 7 是参照文献[14]提出的苯羟基化可能的光 [J]. Angewandte Chemie, 2003, 115(40): 5087-5090.
[7] Acharyya S S, Ghosh S, Tiwari R, et al. Synergistic effect between
催化机理。
ultrasmall Cu( Ⅱ ) oxide and CuCr 2O 4 spinel nanoparticles in
selective hydroxylation of benzene to phenol with air as oxidant [J].
ACS Catalysis, 2015, 5(5): 2850-2858.
[8] Wang Y, Wang X, Antonietti M. Polymeric graphitic carbon nitride as
a heterogeneous organocatalyst: from photochemistry to multipurpose
catalysis to sustainable chemistry[J]. Angewandte Chemie International
Edition, 2012, 51(1): 68-89.
[9] Dai Xiaoqiang (戴小强), Zhu Yabo (朱亚波), Xu Xiaoliang (许孝
良), et al. Photocatalysis with g-C 3N 4 applied to organic synthesis
[J]. Chinese Journal of Organic Chemistry (有机化学), 2017, 37(3):
577-585.
[10] Chen Xiufang, Zhang Jinshui, Fu Xianzhi, et al. Fe-g-C 3N 4-Catalyzed
图 7 苯羟基化可能的光催化机理 oxidation of benzene to phenol using hydrogen peroxide and visible
light [J]. Journal of the American Chemical Society, 2009, 131(33):
Fig. 7 Possible photocatalytic mechanism of hydroxylation of
benzene under visible light 11658-11659.
[11] Verma S, Nasir Baig R B, Nadagouda M N, et al. Photocatalytic C–H
activation of hydrocarbons over VO@g-C 3N 4 [J]. ACS Sustainable
H 2 O 2 在 VO@g-C 3 N 4 -T 催化剂表面被光照产生
Chemistry & Engineering, 2016, 4(4): 2333-2336.
羟基自由基,在石墨化氮化碳表面通过非共价键作 [12] Verma S, Baig R B N, Han C, et al. Oxidative esterification via
用及催化剂的作用,苯分子的 C—H 得到活化。产 photocatalytic C—H activation[J]. Green Chemistry, 2016, 18(1):
251-254.
生的羟基自由基和活化的苯分子作用形成了苯酚。 [13] Yang S, Gong Y, Zhang J, et al. Exfoliated graphitic carbon nitride
nanosheets as efficient catalysts for hydrogen evolution under visible
3 结论 light [J]. Advanced Materials, 2013, 25(17): 2452-2456.
[14] Verma S, Nasir Baig R B, Nadagouda M N, et al. Hydroxylation of
采用简单的尿素热解、超声液相剥离及与乙酰 benzene via C—H activation using bimetallic CuAg@g-C 3N 4 [J].
ACS Sustainable Chemistry & Engineering, 2017, 5(5): 3637-3640.
丙酮氧钒络合的方法制备了 VO@g-C 3 N 4 -T 催化剂。 [15] Sing K S W, Everett D H, et al. Reporting physisorption data for
并用于可见光下催化苯羟基化反应,具有较高的光 gas/solid systems with special reference to the determination of