Page 223 - 《精细化工》2021年第10期
P. 223
第 10 期 蒋宇佳,等: 深共融溶剂催化制备二酯类化合物 ·2153·
2.4 深共融溶剂的表征 醇的反应活性较高。由于生成的二酯类化合物与
对 DESs(氯化胆碱/对甲苯磺酸)进行了 FTIR DESs 明显分层,移除上层二酯产品后剩余 DESs 能
表征,见图 2。如图 2 所示,与对甲苯磺酸的 FTIR 够循环使用,避免了废酸的排放,该方法具有环境
光谱相比,形成 DESs 后磺酸基上氢的谱峰大幅度 友好、操作简便等优点,在工业上具有很好的应用
向高波数移动,同时强度增加,谱带变宽,说明 DESs 前景。
中存在分子间氢键的相互作用。
参考文献:
[1] MULAY A, RATHOD V K. Esterification of maleic acid and butanol
using cationic exchange resin as catalyst[J]. Journal of Chemical
Sciences, 2017, 129(11): 1713-1720.
[2] LENG Y, JIANG P P, WANG J. A novel Brønsted acidic
heteropolyanion-based polymeric hybrid catalyst for esterification[J].
Catalysis Communications, 2012, 25: 41-44.
[3] RAJABI F, WILHELM C, THIEL W R. A Brønsted acidic, ionic
liquid containing, heteropolyacid functionalized polysiloxane
network as a highly selective catalyst for the esterification of
dicarboxylic acids[J]. Green Chemistry, 2020, 22(14): 4438-4444.
[4] FAREGHI-ALAMDARI R, NIRI M N, HAZARKHANI H.
Synthesis and characterization of a new hydroxyl functionalized
diacidic ionic liquid as catalyst for the preparation of diester
plasticizers[J]. Journal of Molecular Liquids, 2017, 227: 153-160.
[5] ROMERO A, SANTOS A, TOJO J, et al. Toxicity and
图 2 DESs 与对甲苯磺酸的 FTIR 谱图 biodegradability of imidazolium ionic liquids[J]. J Hazard Mater,
Fig. 2 FTIR of DESs and p-toluenesulfonic acid 2008, 151(1): 268-273.
[6] PLECHKOVA N V, SEDDON K R. Applications of ionic liquids in
the chemical industry[J]. Chem Soc Rev, 2008, 37(1): 123-150.
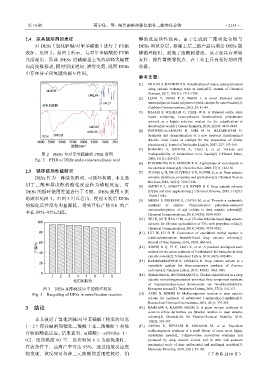
2.5 循环使用性能研究 [7] ZHANG Q H, DE OLIVEIRA V K, ROYER S, et al. Deep eutectic
DESs 作为一种绿色溶剂,可循环利用,本文选 solvents: Syntheses, properties and applications[J]. Chemical Society
Reviews, 2012, 41(21): 7108-7146.
用丁二酸和异戊醇的酯化反应作为模板反应,对 [8] SMITH E L, ABBOTT A P, RYDER K S. Deep eutectic solvents
DESs 的循环使用性能进行了考察,DESs 使用 8 次 (DESs) and their applications[J]. Chemical Reviews, 2014, 114(21):
11060-11082.
的效果见图 3。由图 3 可以看出,使用 8 次后 DESs [9] MESSA F, PERRONE S, CAPUA M, et al. Towards a sustainable
的催化活性没有明显降低,得到目标产物Ⅱb 的产 synthesis of amides: Chemoselective palladium-catalysed
aminocarbonylation of aryl iodides in deep eutectic solvents[J].
率在 88%~95%之间。 Chemical Communications, 2018, 54(58): 8100-8103.
[10] WU K, SU T, HAO D M, et al. Choline chloride-based deep eutectic
solvents for efficient cycloaddition of CO 2 with propylene oxide[J].
Chemical Communications, 2018, 54(69): 9579-9582.
[11] LIU W, LU G H. Carbonation of epoxidized methyl soyates in
tetrabutylammonium bromide-based deep eutectic solvents[J].
Journal of Oleo Science, 2018, 67(5): 609-616.
[12] XIONG X Q, YI C, LIAO X, et al. A practical multigram-scale
method for the green synthesis of 5-substituted-1H-tetrazoles in deep
eutectic solvent[J]. Tetrahedron Letters, 2019, 60(5): 402-406.
[13] KESHAVARZIPOUR F, TAVAKOL H. Deep eutectic solvent as a
recyclable catalyst for three-component synthesis of β-amino
carbonyls[J]. Catalysis Letters, 2015, 145(4): 1062-1066.
[14] SHAABANI A, HOOSHMAND S E. Choline chloride/urea as a deep
eutectic solvent/organocatalyst promoted three-component synthesis
of 3-aminoimidazo-fused heterocycles via Groebke-Blackburn-
图 3 DESs 在酯化反应中的循环利用 Bienayme process[J]. Tetrahedron Letters, 2016, 57(3): 310-313.
Fig. 3 Recycling of DESs in esterification reaction [15] AZIZI N, EDRISI M. Multicomponent reaction in deep eutectic
solvent for synthesis of substituted 1-aminoalkyl-2-naphthols[J].
Research on Chemical Intermediates, 2016, 43(1): 379-385.
3 结论 [16] BAHRANI A, KARIMI-JABERI Z. A green one-pot synthesis of
α-amino nitrile derivatives via Strecker reaction in deep eutectic
solvents[J]. Monatshefte für Chemie-Chemical Monthly, 2018,
本文报道了氯化胆碱/对甲苯磺酸(物质的量比 150(2): 303-307.
1∶2)深共融溶剂催化二羧酸(或二羧酸酐)类化 [17] ARYAN R, BEYZAEI H, NOJAVAN M, et al. Expedient
multicomponent synthesis of a small library of some novel highly
合物的酯化反应。结果表明,n(羧酸)∶n(DESs)=1∶
substituted pyrido[2, 3-d]pyrimidine derivatives mediated and
0.2、反应温度 90 ℃、反应时间 6 h 为最优条件, promoted by deep eutectic solvent and in vitro and quantum
mechanical study of their antibacterial and antifungal activities[J].
在该条件下,最高产率可达 95%,通过拓展反应底
Molecular Diversity, 2018, 23(1): 93-105.
物发现,该反应对各种二元羧酸的适用性较好,伯 (下转第 2160 页)