Page 83 - 201812
P. 83
第 12 期 刘玉娟,等: CeO 2 形貌对甲醇水蒸汽重整 CuO/CeO 2 催化剂的影响 ·2051·
[30]
纳米棒状 CuO-CeO 2 催化材料相比,催化活性基 貌对甲醇水蒸汽重整制氢 CuO/CeO 2 催化剂催化性
本相接近,说明纳米棒状 CuO/CeO 2 催化材料对甲 能的影响。结果表明,CeO 2 形貌与催化剂的结构、
醇水蒸汽重整制氢反应具有良好的催化活性。 性质和性能密切相关,纳米棒状 CeO 2 表面存在较多
的晶格缺陷和氧空穴,CeO 2 与 CuO 相互作用较强,
使得 CuO/CeO 2 -rod 催化剂表相 Cu 含量增加,表相
Cu 物种的还原温度较低,催化活性较好。当反应温
度为 260 ℃、水醇物质的量比为 1.2、甲醇气体空速
–1
为 800 h 时,CuO/CeO 2 -rod 催化甲醇转化率可达到
100%。通过改变焙烧气氛合成不同形貌的催化材料
方法简单,成本较低,易于工业化大规模操作。为
了合成出形貌单一和尺寸可控的纳米材料,如何选
择焙烧过程中的条件(如焙烧温度,被烧时间等)
有待进一步研究。
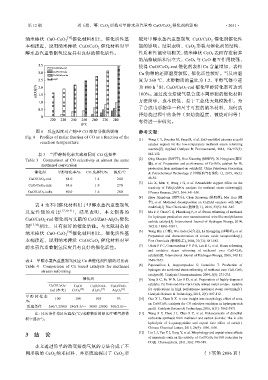
图 8 反应温度对产物中 CO 摩尔分数的影响 参考文献:
Fig. 8 Profiles of molar fraction of CO as a function of the [1] Wang C Y, Boucher M, Yang M, et al. ZnO-modified zirconia as gold
reaction temperature catalyst support for the low-temperature methanol steam reforming
reaction[J]. Applied Catalysis B: Environmental, 2014, 154/155(5):
表 3 当甲醇转化率大致相同时 CO 选择性 142-152.
Table 3 Comparison of CO selectivity at almost the same [2] Qing Shaojun (庆绍军), Hou Xiaoning (侯晓宁), Xi Hongjuan (郗宏
methanol conversion 娟), et al. Preparation and performance of Cu-SiO 2 catalyst for H 2
production from methanol on vehicle[J]. China Petroleum Processing
催化剂 甲醇转化率/% CO 选择性/% 温度/℃ & Petrochemical Technology (中国炼油与石油化工), 2015, 46(1):
CuO/CeO 2-rod 84.0 1.4 240 48-52.
[3] Liu X, Men Y, Wang J G, et al. Remarkable support effect on the
CuO/CeO 2-mix 84.6 1.9 270
reactivity of Pt/In 2O 3/MOx catalysts for methanol steam reforming[J].
CuO/CeO 2-cube 80.0 1.6 280 J Power Sources, 2017, 364: 341-350.
[4] Zhou Xingdong (周性东), Chen Xiaorong (陈晓蓉), Mei Hua (梅
华), et al. Methanol decomposition on CuZnAl catalysts with MgO
表 4 为不同催化材料用于甲醇水蒸汽重整制氢 modified[J]. Fine Chemicals (精细化工), 2016, 33(5): 541-545.
反应性能的对比 [17,30-31] ,结果表明,本文制备的 [5] Ma Y F, Guan G Q, Phanthong P, et al. Steam reforming of methanol
for hydrogen production over nanostructured wire-like molybdenum
CuO/CeO 2 -rod 催化剂与文献的 CuO/ZnO-Al 2 O 3 催化
carbide catalyst[J]. International Journal of Hydrogen Energy, 2014,
剂 [17,30] 相比,具有较好的催化活性。与文献制备的 39(33): 18803-18811.
[30] 催化材料相比,催化活性基 [6] Wang Bin (王彬), Wu Jieda (吴介达), Li Xiongping (李雄平), et al.
纳米棒状 CuO-CeO 2
Preparation and characterization of cerium oxide nanoparticles[J].
本相接近,说明纳米棒状 CuO/CeO 2 催化材料对甲 Fine Chemicals (精细化工), 2004, 21(12): 881-883.
醇水蒸汽重整制氢反应具有良好的催化活性。 [7] Udani P P C, Gunawardana P V D S, Lee H C, et al. Steam reforming
and oxidative steam reforming of methanol over CuO-CeO 2
catalysts[J]. International Journal of Hydrogen Energy, 2009, 34(18):
表 4 甲醇水蒸汽重整制氢反应 Cu 基催化剂性能的对比表 7648-7655.
Table 4 Comparison of Cu based catalysts for methanol [8] Papavasiliou J, Avgouropoulos G, Ioannides T. Production of
stream reforming hydrogen via combined steam reforming of methanol over CuO-CeO 2
catalysts[J]. Catalysis Communications, 2004, 5(5): 231-235.
催化剂 [9] Yang S C, Su W N, Lin S D, et al. Preparation of highly dispersed
CuO/CeO 2- CuO/ CuO/ZnO- CuO/ZnO- catalytic Cu from rod-like CuO-CeO 2 mixed metal oxides:suitable
rod (本文) CeO 2 [30] Al 2O 3 [17] Al 2O 3 [31] for applications in high performance methanol steam reforming[J].
Catalysis Science & Technology, 2012, 2(4): 807-812.
甲醇转化率 100 100 100 93 [10] Guo X L, Zhou R X. A new insight into morphology effect of ceria
/% on CuO/CeO 2 catalysts for CO selective oxidation in hydrogen-rich
反应条件 260/1.2/800 240/1.5/— 300/1.2/800 300/2.0/—
gas[J]. Catalysis Science & Technology, 2016, 6(11): 3862-3871.
注:反应条件指反应温度(℃)/水醇物质的量比/甲醇气体体 [11] Wang S P, Zhou J J, Zhao S Y, et al. Enhancements of dimethyl
积空速(h )。 carbonate synthesis from methanol and carbon dioxide: The in situ
–1
hydrolysis of 2-cyanopyridine and crystal face effect of ceria[J].
Chinese Chemical Letters, 2015, 26(9): 1096-1100.
3 结 论 [12] Liu L J, Yao Z J, Deng Y, et al. Morphology and crystal-plane effects
of nanoscale ceria on the activity of CuO/CeO 2 for NO reduction by
CO[J]. Chemcatchem, 2011, 3(6): 978-989.
本文通过简单的改变焙烧气氛的方法合成了不
同形貌的 CeO 2 纳米材料,并系统地探讨了 CeO 2 形 (下转第 2086 页)