Page 46 - 201907
P. 46
·1292· 精细化工 FINE CHEMICALS 第 36 卷
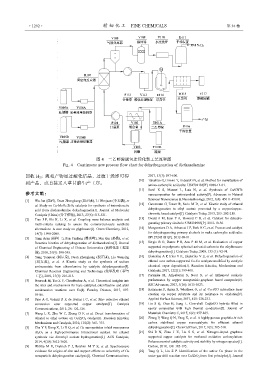
图 4 二乙醇胺脱氢连续化新工艺流程图
Fig. 4 Continuous new process flow chart for dehydrogenation of diethanolamine
回收 H 2 ;液相产物经过酸化结晶、过滤干燥即可得 2017, 53(3): 597-600.
[10] Takakiyo G, Hiromi Y, Hideyuki N, et al. Method for manufacture of
到产品,或直接送入草甘膦生产工序。 amino-carboxylic acid salts: US4782183[P]. 1988-11-01.
[11] Sunil K S, Maneet L, Lata N, et al. Synthesis of Cu/CNTs
参考文献: nanocomposites for antimicrobial activity[J]. Advances in Natural
[1] Wu Jun (伍君), Duan Zhengkang (段正康), Li Wenjuan (李文娟), et Sciences Nanoscience & Nanotechnology, 2012, 3(4): 45011-45010.
al. Study on Cu-MoO 3-ZrO 2 catalysts for synthesis of iminodiacetic [12] Carotenuto G, Tesser R, Serio M D, et al. Kinetic study of ethanol
acid from diethanolamine dehydrogenatio[J]. Jounral of Molecular dehydrogenation to ethyl acetate promoted by a copper/copper-
Catalysis (China) (分子催化), 2013, 27(6): 515-521. chromite based catalyst[J]. Catalysis Today, 2013, 203: 202-210.
[2] Tian J P, Shi H, Li X, et al. Coupling mass balance analysis and [13] Dvaid A M, Juan P A, Howard C B, et al. Catalyst for dehydro-
multi-criteria ranking to assess the commercial-scale synthetic genating primary alcohols: US8298985[P]. 2012-10-30.
alternatives: A case study on glyphosate[J]. Green Chemistry, 2012, [14] Morgenstern D A, Arhancet J P, Berk H C, et al. Process and catalyst
14(7): 1990-2000. for dehydrogenating primary alcohols to make carboxylic acid salts:
[3] Yang Asan (杨阿三), Pan Yanfeng (潘炎峰), Sun Qin (孙勤), et al. EP1272451B1[P]. 2012-08-01.
Reaction kinetics of dehydrogenation of diethanolamine[J]. Journal [15] Sérgio B O, Danns P B, Ana P M M, et al. Evaluation of copper
of Chemical Engineering of Chinese Universities (高校化学工程学 supported on polymeric spherical activated carbon in the ethylbenzene
报), 2010, 24(4): 590-595. dehydrogenation[J]. Catalysis Today, 2008, 133 (1): 92-98.
[4] Yang Yunquan (杨运泉), Duan Zhengkang (段正康), Liu Wenying [16] Ekaterina A P, Irina V K, Ekaterina V E, et al. Dehydrogenation of
(刘文英), et al. A Kinetic study on the synthesis of sodium ethanol over carbon-supported Cu-Co catalysts modified by catalytic
aminoacetate from ethanolamine by catalytic dehydrogenation[J]. chemical vapor deposition[J]. Reaction Kinetics, Mechanisms and
Chemical Reaction Engineering and Technology (化学反应工程与 Catalysis, 2017, 122(1): 399-408.
工艺), 2001, 17(3): 210-215. [17] Paramita M, Arjyabaran S, Noor S, et al. Enhanced catalytic
[5] Neurock M, Tao Z Y, Chemburkar A, et al. Theoretical insights into performance by copper nanoparticle-graphene based composite[J].
the sites and mechanisms for base catalyzed esterification and aldol RSC Advances, 2013, 3(16): 5615-5623.
condensation reactions over Cu[J]. Faraday Discuss, 2017, 197: [18] Katarzyna P, Agata S, Wiesława O, et al. Cu-rGO subsurface layer
59-86. creation on copper substrate and its resistance to oxidation[J].
[6] Sato A G, Volanti D P, de Freitas I C, et al. Site- selective ethanol Applied Surface Science, 2017, 421: 228-233.
conversion over supported copper catalysts[J]. Catalysis [19] Liu S Q, Zhao B, Jiang L. Core-shell Cu@rGO hybrids filled in
Communications, 2012, 26: 122-126. epoxy composites with high thermal conduction[J]. Journal of
[7] Wang L X, Zhu W C, Zheng D F, et al. Direct transformation of Materials Chemistry C, 2017, 6(2): 257-265.
ethanol to ethyl acetate on Cu/ZrO 2 catalyst[J]. Reaction Kinetics, [20] Zhang P, Wang Q N, Yang X, et al. A highly porous graphitic-N rich
Mechanisms and Catalysis, 2010, 101(2): 365- 375. carbon stabilized copper nanocatalysts for efficient ethanol
[8] Zhu Y F, Kong X, Li X Q, et al. Cu nanoparticles inlaid mesoporous dehydrogenation[J]. ChemCatChem, 2017, 9(3): 505-510.
Al 2O 3 as a high-performance bifunctional catalyst for ethanol [21] Shi R N, Zhao J X, Liu S S, et al. Nitrogen-doped graphene
synthesis via dimethyl oxalate hydrogenation[J]. ACS Catalysis, supported copper catalysts for methanol oxidative carbonylation:
2014, 4(10): 3612-3620. Enhancement of catalytic activity and stability by nitrogen species[J].
[9] Witzke M E, Dietrich P J, Ibrahim M Y S, et al. Spectroscopic Carbon, 2018, 130: 185-195.
evidence for origins of size and support effects on selectivity of Cu [22] Tang Q L, Liu Z P. Identification of the active Cu phase in the
nanoparticle dehydrogenation catalysts[J]. Chemical Communications, water-gas shift reaction over Cu/ZrO 2 from first principles[J]. Journal