Page 149 - 精细化工2019年第8期
P. 149
第 8 期 刘 通,等: l-薄荷醇的合成工艺研究 ·1637·
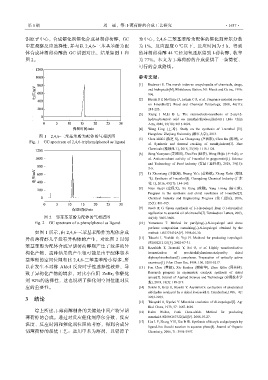
铝原子中心,合成催化剂催化合成异胡薄荷醇,GC 为中心,2,4,6-三苯基苯酚为配体的催化剂摩尔分数
中控观察反应选择性,并与以 2,4,6-三苯基苯酚为配 为 1%,反应温度 0 ℃以下,反应时间为 5 h,得到
体合成异胡薄荷醇的 GC 谱图对比,结果如图 1 和 的异胡薄荷醇 44 ℃经加氢还原得到 l-薄荷醇,收率
图 2。 为 77%。本文为 1-薄荷醇的合成提供了一条简便、
可行的合成路线。
参考文献:
[1] Budavari S. The merck index-an encyclopedia of chemicals, drugs,
and biologicals[M].Whitehouse Station, NJ: Merck and Co inc, 1996:
996.
[2] Bhatia S P, McGinty D, Letizia C S, et al. Fragrance material review
on l-menthol[J]. Food and Chemical Toxicology, 2008, 46(11):
218-223.
[3] Xiang J M,Li B L. The stereoselectivesynthesis of 2-aryl-2-
hydroxybutanoic acid via menthylchiralauxiliaries[J]. Helv Chim
Acta, 2010, 93(10): 2015-2022.
[4] Wang Ling (王玲). Study on the synthesis of l-menthol [D].
图 1 2,4,6-三苯基苯酚为配体的气相谱图 Hangzhou: Zhejiang University (浙江大学), 2013.
Fig. 1 GC spectrum of 2,4,6-triphenylphenol as ligand [5] Chen Zhifei (陈芝飞), Lu Changtong (芦昶彤), Chen Jin (陈瑨), et
al. Synthesis and thermal cracking of menthylnitrate[J]. Fine
Chemicals (精细化工), 2016, 33(10): 1118-1124.
[6] Jiang Yuanyuan (姜圆圆), ZhaoYan (赵岩), Wang Shijie (王士杰), et
al. Anticonvulsant activity of l-menthol in peppermint[J]. Science
and Technology of Food Industry (食品工业科技), 2018, 39(15):
5-9.
[7] Li Zhenxiang (李振翔), Huang Yu’e (黄裕娥), Zheng Xufei (郑旭
飞). Synthesis of l-menthol[J]. Guangdong Chemical Industry (广东
化工), 2016, 43(17): 144-145.
[8] Nian Baoyi (念保义), Xu Gang (徐刚), Yang Lirong (杨立荣).
Progress in the synthesis and chiral resolution of l-menthol[J].
Chemical Industry and Engineering Progress (化工进展), 2006,
25(4): 401-405.
[9] Jacob R G. Green synthesis of (–)-isopulegol from (+)-citronellal:
application to essential oil of citronella[J]. Tetrahedron Letters, 2003,
图 2 邻苯基苯酚为配体的气相谱图 44(18): 3605-3608.
Fig. 2 GC spectrum of o-phenylphenol as ligand [10] Yamamoto T. Method for purifying(–)-N-isopulegol and citrus
perfume composition containing(–)-N-isopulegol obtained by the
如图 1 所示,由 2,4,6-三苯基苯酚作为配体合成 method: US5773410A[P]. 1998-06-30.
异胡薄荷醇几乎没有异构体的产生,对比图 2 以邻 [11] Takeshi I, Yoshiki O, Yoji H. Methord for producing isopulegol:
JP2002212121[P]. 2002-07-31.
苯基苯酚为配体合成异胡薄荷醇则产生了较多的异 [12] Kazuhide T, Tsuneaki Y, Sei O, et al. Highly enantioselective
构化产物,这种结果的产生很可能是由于配体邻苯 isomerization of prochiralallylaminescatalyzedby chiral
diphosphinerhodium(I) complexes. Preparation of optically active
基苯酚的空间位阻相比 2,4,6-三苯基苯酚小得多,所
enamines[J]. J Am Chem Soc, 1984, 106, 5208-5217.
以在发生不对称 Aldol 反应时手性选择性较差,导 [13] Fan Chao (樊超), Xie Jianhua (谢建华), Zhou Qilin (周其林).
致了异构化产物的增多,对比小位阻 ZnBr 2 作催化 Research progress in asymmetric catalytic synthesis of chiral
spices[J]. Journal of Applied Science and Technology (应用技术学
剂 92%的选择性,这也说明了催化剂空间位阻对反 报), 2018, 18(3): 189-219.
应的重要性。 [14] Soichi S, Keiji U, Hisashi Y. Asymmetric cyclization of unsaturated
aldehydes catalyzed by a chiral lewisacid[J]. Tetrahedron,1986,42:
3 结论 2203-2209.
[15] Takayuki O, Kyohei Y. Microbial resolution of dl-isopulegol[J]. Agr
BioI Chem, 1973, 37: 1687-1689.
综上所述,l-薄荷醇制备的关键是中间产物异胡 [16] Kuhn Walter, Funk Hans-ulrich. Method for producing
薄荷醇的合成。通过对反应催化剂摩尔分数,反应 menthol:US2006167322(A1)[P]. 2006-07-27.
[17] Liu L F, Zhang Y H, Xin B W. Synthesis of biaryls and polyaryls by
温度、反应时间和催化剂位阻的考察,得到合成异
ligand-free Suzuki reaction in aqueous phase[J]. Journal of Organic
胡薄荷醇的最优工艺:选用甲苯为溶剂,以铝原子 Chemistry, 2006, 71: 3994-3997.