Page 163 - 《精细化工》2020年第2期
P. 163
第 2 期 孙苗苗,等: 可注射 CS/PLLA-SA 复合水凝胶的制备及载药性 ·365·
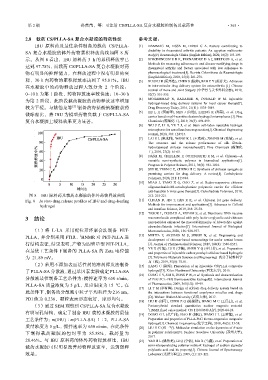
2.8 载药 CS/PLLA-SA 复合水凝胶的释药性能 参考文献:
IBU 原料药及最佳条件制备的载药 CS/PLLA- [1] HAMMAG M, EISSA M, DAWA G A. Factors contributing to
disability in rheumatoid arthritis patients: An egyptian multicenter
SA 复合水凝胶的体外药物累积释放曲线如图 8 所
study[J]. Reumatología Clínica (English Edition), 2020, 16(2): 103-109.
示。从图 8 看出,IBU 原料药 3 h 的累积释放率已 [2] RINCININCON E R R, FERNANDEZ D A J, BELTRAB A, et al.
Methods for measuring adherence to oral disease-modifying drugs in
达到 97.78%,而载药 CS/PLLA-SA 复合水凝胶对药 rheumatoid arthritis and factors associated with low adherence to
物有明显的控释能力,在释放过程中没有明显的突 pharmacological treatment[J]. Revista Colombiana de Reumatología
(English Edition), 2018, 25(4): 261-270.
释,30 h 内药物的累积释放率达到了 95.81%。IBU [3] RUAN J H (阮基浩), CHEN S (陈帅), FAN C Y (范存义). Advances
在水凝胶中的药物释放过程大致分为 2 个阶段: in intra-articular drug delivery system for osteoarthritis [J]. Chinese
Journal of Bone and Joint Surgery (中华骨与关节外科杂志), 2019,
0~10 h 为第 1 阶段,药物释放速率较快速;10~30 h 12(7): 551-555.
为第 2 阶段,此阶段载药凝胶的药物释放速率增加 [4] MOHAMMAD N, BAHAREH N, DONALD W M. Injectable
hydrogel-based drug delivery systems for local cancer therapy[J].
较为平缓。与姚伯龙等 [21] 制备的海藻酸钠凝胶载药 Drug Discovery Today, 2016, 21(11): 1835-1849.
[5] HE L H (何丽华), MIN J (闵洁), ZHENG R (郑荣), et al. Drug
微球相比,将 IBU 为模型药物负载于 CS/PLLA-SA carrier based on pH-sensitive dextran hydrogel microspheres [J]. Fine
复合水凝胶上缓释效果更为显著。 Chemicals (精细化工), 2019, 36(3): 494-499.
[6] WU J Z, LI G, YE T J, et al. Stem cell-laden injectable hydrogel
microspheres for cancellous bone regeneration[J]. Chemical Engineering
Journal, 2020, 393: 124715.
[7] LAI S L (来水利), WANG K L (王克玲), ZHANG M (张敏), et al.
The structure and the release performance of silk fibroin-
hydroxypropyl chitosan microspheres[J]. Fine Chemicals (精细化
工), 2010, 27(2): 61-65.
[8] DASH M, CHIELLINI F, OTTENBRITE R M, et al. Chitosan—A
versatile semi-synthetic polymer in biomedical applications[J].
Progress in Polymer Science, 2011, 36(8): 981-1014.
[9] SHI W, YERN C C, CHENG H C. Synthesis of chitosan aerogels as
promising carriers for drug delivery: A review[J]. Carbohydrate
Polymers, 2020, 231: 115744.
[10] MIAO J, YANG X Q, GAO Z, et al. Redox-responsive chitosan
oligosaccharide-SS-octadecylamine polymeric carrier for efficient
anti-hepatitis b virus gene therapy[J]. Carbohydrate Polymers, 2019,
图 8 IBU 原料药及载药水凝胶的体外药物释放曲线 212: 215-221.
Fig. 8 In vitro drug release profiles of IBU and drug-loading [11] CHUAN D, JIN T, FAN R R, et al. Chitosan for gene delivery:
hydrogel Methods for improvement and applications[J]. Advances in Colloid
and Interface Science, 2019, 268: 25-38.
[12] TASOK L, IRSHAD A, RUPAM S, et al. Bicistronic DNA vaccine
3 结论 macromolecule complexed with poly lactic-co-glycolic acid-chitosan
nanoparticles enhanced the mucosal immunity of labeorohita against
edwardsiellatarda infection[J]. International Journal of Biological
(1)将 L-LA 采用配位开环聚合法制备 PET- Macromolecules, 2020, 156: 928-937.
1
PLLA,并分别采用 FTIR、 HNMR 对 PET-PLLA 进 [13] MEHTA P, ARSHAD M S, SINGH N, et al. Engineering and
development of chitosan-based nanocoatings for ocular contact lenses
行结构表征,结果表明,产物为四臂星型 PET-PLLA, [J]. Journal of Pharmaceutical Sciences, 2019, 108(4): 1540-1551.
[14] YE X (叶旭), LI X (李娴), SHEN Y Q (申月琴), et al. Preparation
在最优工艺条件下制备的 PLLA-SA 的 Zeta 电位值
and properties of injectable carboxymethyl cellulose based hydrogels
为–21.89 mV。 [J]. Polymeric Materials Science and Engineering( 高分子材料科学
与工程), 2019, 35(8): 75-81.
(2)采用不添加表面活性剂的溶剂挥发法制备 [15] KANG C (康纯). Preparation of an injectable CS/PLLA composite
了 PLLA-SA 分散液,通过单因素实验确定 PLLA-SA hydrogel[D]. Xi'an: Northwest University (西北大学), 2016.
[16] GONG C Y, SHI S, DONG P W, et al. Synthesis and characterization
分散液最佳制备工艺条件为:搅拌速率为 650 r/min, of PEG-PCL-PEG thermosensitive hydrogel[J]. International Journal
PLLA-SA 质量浓度为 5 g/L,反应温度为 15 ℃。在 of Pharmaceutics, 2009, 365(1/2): 89-99.
[17] LI Y M (李有梅). Design of effcient drug delivery system based on
此条件下,制备的分散液中粒子平均粒径为 216 nm, the interactions between functional copolymer micelles and drugs
[D]. Wuhan: Wuhan University (武汉大学), 2017.
PDI 值为 0.236,颗粒表面形态较好,球形均匀。
[18] LIU K (刘可), CHEN Z Q (陈智群), WANG M C (王民昌), et al.
(3)通过 SEM 观察到 CS/PLLA-SA 复合水凝胶 Pentaerythritol standard quantitative nuclear magnetic material
1 HNMR fixed value method: CN 111060548 A[P]. 2020-04-24.
有明显孔结构,确定了制备 IBU 载药水凝胶的最佳 [19] DONG G L (董广利), HAO H (郝红), WANG J L (王君莲), et al.
工艺条件为:m(IBU)∶m(PLLA-SA)=1∶2,PLLA-SA Preparation and properties of PLLA-PEG thermo-responsive composite
hydrogel[J]. Chemical Engineering (化学工程), 2018, 46(5): 53-58.
质量浓度为 5 g/L,搅拌速率为 650 r/min,在此条件 [20] LIU Y C (刘一岑). Molecular simulation on the dynamics of θ state
下 制得载药凝胶的包封率为 85.38%,载药量为 in polymer solutions[D]. Suzhou: Soochow University (苏州大学),
2017.
28.46%。与 IBU 原料药的体外药物释放相比,IBU [21] YAO B L (姚伯龙), LI Q (李倩), MA X (马鑫), et al. Preparation of
载药水凝胶可以明显改善药物释放速率,达到缓释 semi-interpenetrating polymer network hydrogel of sodium alginate/
polylactic acid and its property[J]. Chinese Journal of Spectroscopy
效果。 Laborator (光谱实验室), 2009, (1): 119-121.